Sources of Bromine
Natural sources for bromine are chloride sources such as natural salt stocks and seawater where bromides are accompanying the chlorides. Industrial sources for bromine recovery are quench waters of waste incinerators (coming from the flame retardants) and effluents of industrial bromination processes.
The reactions to form the a.m. chemicals may generate hydrogen bromide as a by-product:
R-H+Br2 => R-Br+HBr
In this case no more than half of the bromine added ends up in the product, the rest being released as hydrogen bromide gas and as dissolved bromides (often rejected in washing solutions). Since bromine is an expensive, widely used raw material, recovery is usually a viable option.
Safety First
Bromine is highly corrosive, toxic, and causes serious chemical burns. 3 ppm in the air are considered immediately dangerous to life and health. Therefore, it is vitally important to take the highest safety precautions when working with bromine.
Accordingly, plants processing bromine have to be made of highly corrosion and diffusion resistant, robust materials. QVF borosilicate glass 3.3 and De Dietrich glass-lined steel are ideal materials for their construction. All measures are taken to avoid any bromine leakage; the equipment fulfills ASME requirements for pressure and tightness of flange connections. For extra safety measures, the vent systems are equipped with bromine scrubbers.
De Dietrich Process Systems' material quality, detailed design and reliable process engineering contribute to the safe handling of bromine.
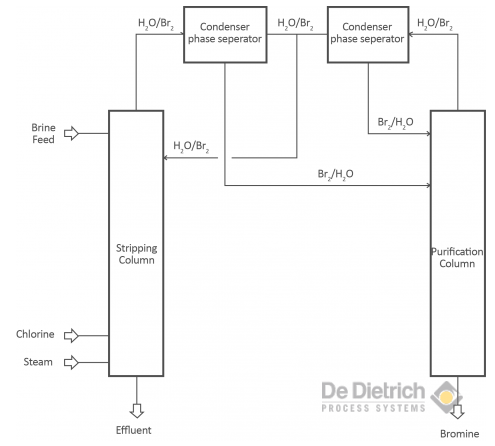
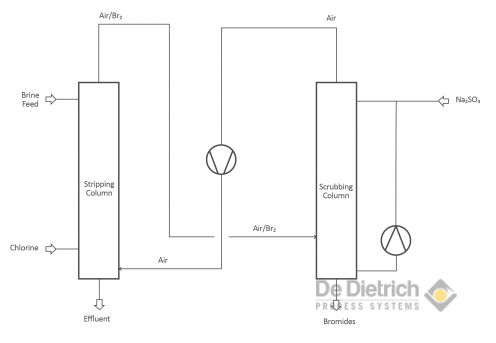
Treating Bromide Containing Brines
There are two different objectives when treating bromide containing brines. One objective is the production of bromine and the other one is to debrominate the brine to get rid of the bromides. The production of bromine from brines as well as the debromination of brines is based on the oxidation of bromide to bromine. In the laboratory scale an oxidating component (concentrated sulfuric acid or acidified KMnO4) is used. In the industrial scale mainly chlorine, but also hydrogen peroxide is used.