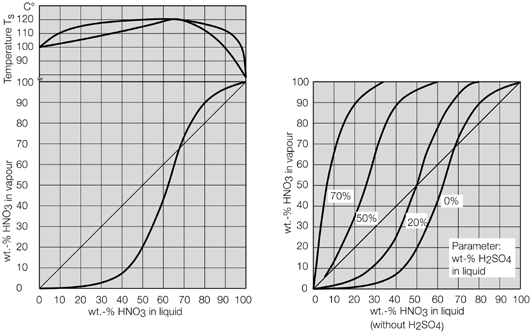
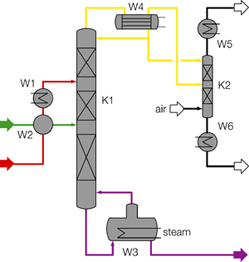
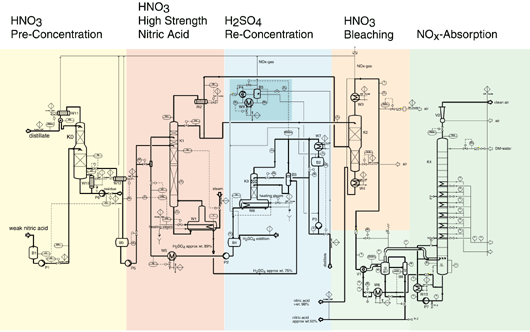
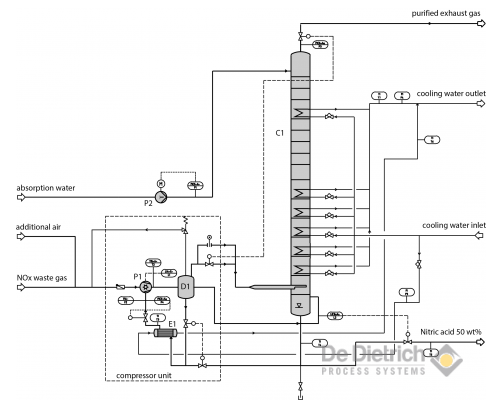
- Systems
- Equipment
- Reactors
- Columns
- Filters and Filter/Dryers
- Powder Handling
- Mixing Systems
- Heat Exchangers
- Sampling Equipment
- Dryers
- Vacuum Pan Dryer
- Vertical Vacuum Dryer
- Horizontal Vacuum Dryer
- Horizontal Vacuum Paddle Dryer / Reactor
- Pilot Plant Filter Dryer and Vacuum Dryer
- Conical Screw Vacuum Dryer
- Conical Screw Mixer
- Conical Screw Cooker
- Cylindro Conical Dryer
- Cylindro Conical Mixer
- Pilot Plant BS-pilotDRY
- Laboratory System BS-miniDRY
- Universal Vacuum Dryers
- Rotary Vacuum Paddle Dryers
- Glass-Lined Conical Dryer-Blenders
- Spherical Dryers
- Agitated Pan Dryers
- Pipes, Valves and Fittings
- Storage Tanks and Receivers
- Reconditioned Equipment
- Evaporators
- Centrifuges
- Inverting Filter Centrifuge HF
- Inverting Filter Centrifuge F
- Horizontal Peeler Centrifuge Pharma
- Horizontal Peeler Centrifuge Chemical
- Vertical Peeler Centrifuge Pharma
- Vertical Peeler Centrifuge Chemical
- Vertical Peeler Centrifuge Gypsum
- Top Discharge Centrifuge Pharma
- Vertical Centrifuge Plasma Fractionation
- Top Discharge Centrifuge Chemical
- Classifying Centrifuge
- Coating Centrifuge
- Horizontal Pilot Plant Centrifuge Pharma
- Horizontal Pilot Plant Centrifuge Chemical
- Vertical Pilot Plant Centrifuge Pharma
- Vertical Pilot Plant Centrifuge Chemical
- Services
- Industries
- About Us
- Resources
- Contact Us